Standard Operation Procedures
Regulations: Title 45 Code of Federal Regulations Part 46 (45 CFR 46) Protection of Human Subjects specifies federal regulations for the conduct of research involving human subjects. All human subjects’ research at Grand Canyon University must be conducted in accordance with 45CFR46. The regulations are available at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html
Completing the Application: All applications must be submitted online through IRBNet using the online forms and written in non-technical terms. It is the investigator's responsibility to provide information about research procedures so that the IRB has a clear understanding of what the research entails. Researchers should keep in mind that the individuals reviewing the application may be unfamiliar with the field of study involved.
If possible, please place your typewritten response directly under each question within the form(s). This is preferable to using a separate sheet during the review process. It is important that you answer every question to ensure that all of the necessary information is included in your application.
- Do not leave items blank in the application, or fail to provide to the IRB any poster, document, or other written communication to subjects.
- Please upload all required forms along with the correct IRB Application into IRBNet.
- All forms and documents are signed through IRBNet’s electronic signature by completing Sign this Package step.
- Your electronic signature is accepted as a legal signature. Remember only your Dissertation Chair can submit the package to GCU IRB.
For Student Researchers: As the primary investigator (PI) it is your responsibility to prepare your IRB application and upload all required documentation into IRBNet. However, only the Dissertation Chair can submit the package to Grand Canyon University’s IRB for review.
In order to do so, you must share the project with your Chair. Student researcher IRB application packages will not be reviewed or approved by the Institutional Review Board until all required electronic signatures are obtained, and your Chair reviews, approves, and submits your application package.
For Faculty / Chairs: Once all documents are uploaded, attached (in final version) and required signatures obtained (Chair, Program Chair etc.) THE CHAIR (or faculty researcher if you are faculty) submits the package for review by completing the mandatory Submit this Package step. Once the Chair “submits the package”, the research cannot be edited or modified unless you contact the IRB Office to unlock the project.
Principal Investigator Responsibilities
- I have reviewed the proposal and will conduct the research study in full compliance with Grand Canyon University (GCU), policies, guidelines, and applicable Federal and State regulations.
- I will not enroll any participants into this research study until the study has been approved, in writing, by the Institutional Review Board (IRB).
- I will employ and assume the responsibility for the informed consent process in order to ensure that potential research participants understand the purpose of the research study, the procedures they are being asked to undergo, the potential risks, benefits, and alternatives of the research study, their rights as a research study participant, and have sufficient time to decide about participating. I will not enroll any participant in the research study or conduct study procedures until such informed consent is obtained.
- I will ensure that research participants are kept fully and promptly informed of any new information that may affect their willingness to continue to participate in the research study.
- I will maintain current and accurate records of research data, outcomes and adverse events to permit an ongoing assessment of the risks / benefits ratio of research participation.
- I will report to the IRB, in a timely manner, all adverse events according to GCU policy and make a reasonable effort to ensure that participants who have suffered an adverse event associated with research participation receive adequate care to correct or alleviate the consequences of the adverse event to extent possible.
- I understand that it is my responsibility to submit the research study in a timely manner (minimally annually) for "Continuing Review" in order to obtain IRB renewal / approval. I am aware that failure to do so will subject my study to suspension or termination.
- I agree to follow the protocol as approved by the IRB. All protocol deviations will be reported to the IRB and documentation of protocol deviations will be kept in the study regulatory files, and if applicable, in the participant's study file.
- I accept responsibility for the study staff and their appropriate conduct and will ensure adequate training for all personnel.
Principal Investigator: Be sure to electronically sign the package prior to having your Chair sign and submit to the IRB.
Approval: Data collection may not begin until student researchers have received approval to conduct the research from IRB. At the same time, research shall not continue beyond the date stated on the approval letter. Research projects involving human subjects can be approved for up to one year in accordance with Federal Regulations.
A study is considered complete when data collection and data analysis are complete.
Step-by-Step Guide for Student Researchers
- New User Registration
- Activate / Reset Account?
- How to Access Study Documents
- Recording a Decision or Recommendation
- How to Access Agendas
New User Registartion
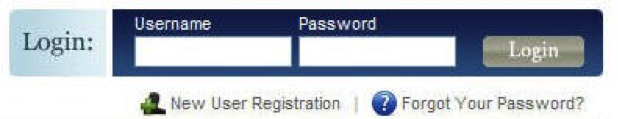
- Log in to www.IRBNet.org
- Click: New User Registration in the upper right hand corner (if you have registered then ignore this step).
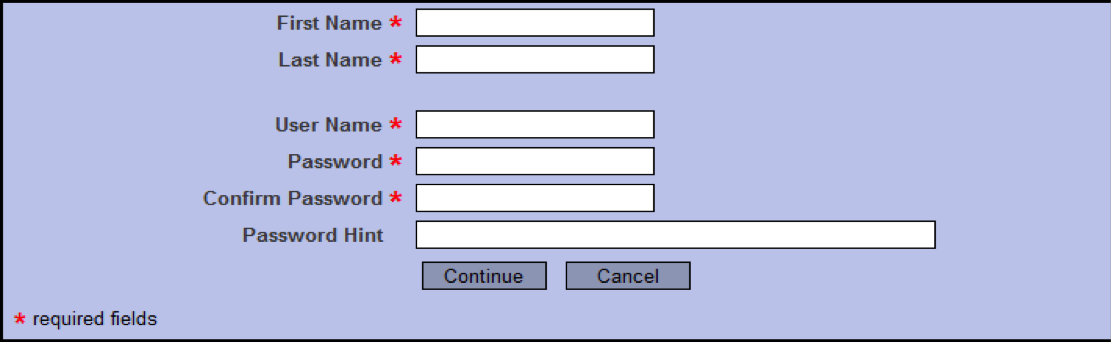
- Enter your legal first and last names in the appropriate fields. (Please use upper case letters when appropriate as you register into the system; the system does not alphabetize names that begin with lower-case letters.)
- Create your User Name and Password; confirm your password, and enter a password hint.
- Select Continue
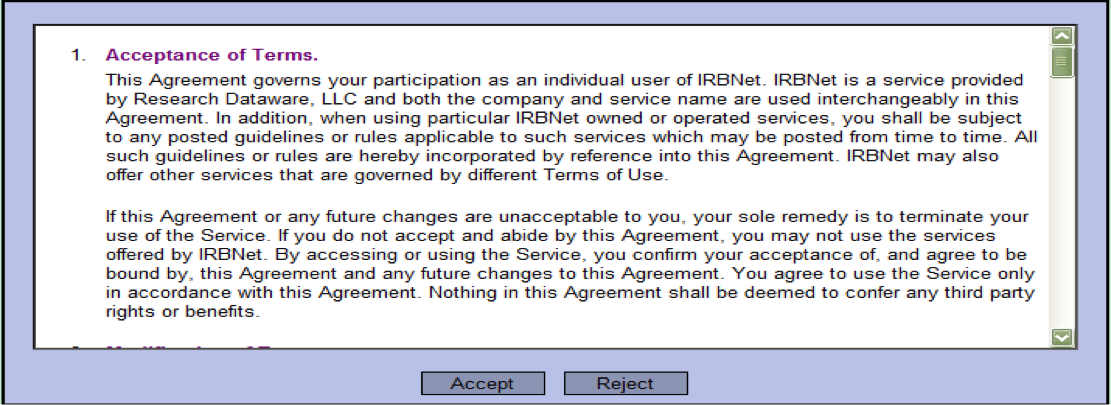
- Accept the Terms of Use
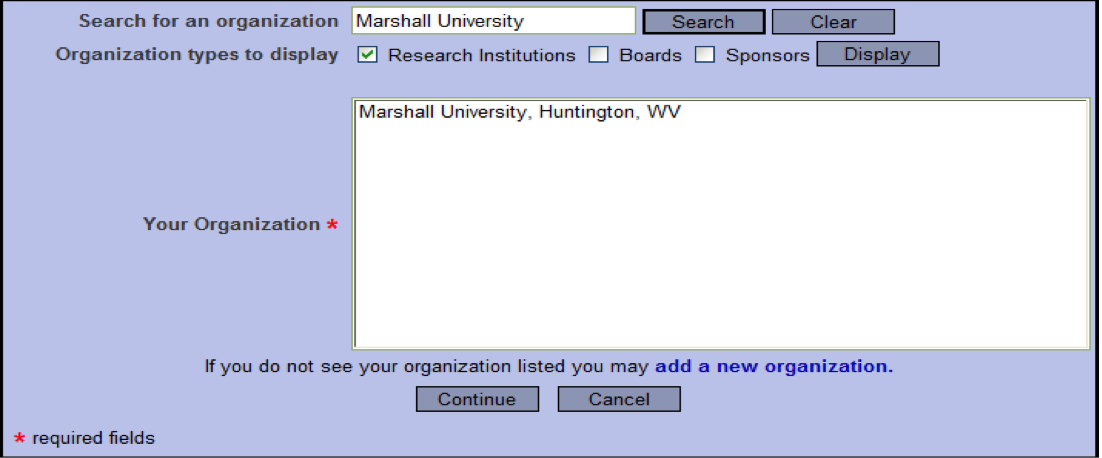
- On the next screen, type “Grand Canyon University” in the “Search for an organization” field and click “Search” OR scroll to and select “Grand Canyon University, Phoenix, AZ” from the list on the screen.
- Select Continue
- On the next screen, enter your telephone number and email address; select Continue
- On the final screen, you will confirm the information you entered. Be sure in the second box, it lists Grand Canyon University, and then select Register.
- You will receive an email from IRBNet with a link that you need to click on to activate your account. Your name will appear on Grand Canyon University’s list after you activate your account. **
Activate / Reset Account?
If you have not received the email authentication from IRBNet it is because either . . .
- The email address was incorrectly typed,
- There is a filter on your email blocking the authentication, or
- You have an alternate account (if so, please create a new email account with Yahoo/ Gmail/ Hotmail).
Try these steps:
Check your spam filters/delete box. If the email is not there, then follow the steps below.
- Log in to www.IRBNet.org
- In the upper right corner of the screen, click on “USER PROFILE”
- At the bottom of the next screen where you see Affiliations, click Edit
- Change your email address
- Enter a new or different fax number
- Click on Save. This will trigger a new activation email that will be sent to your account.
- When you receive the email, click the link in the message. This will take you to the home page of IRBNet, indicating that your account has been successfully activated.
Note: To change email accounts click on the User Profile. This is accessible even if you have not activated your account. Please avoid creating more than one IRBNet account.
Create a New Project
- Log in to www.IRBNet.org (if not already logged in)
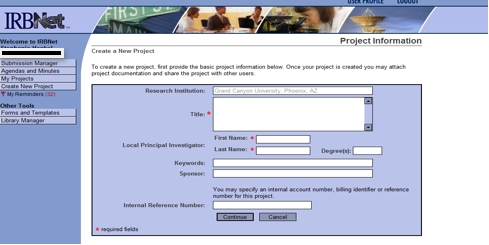
- Select Create New Project
- Enter the Title of your project/study
- Enter your legal first and last name as the Principal Investigator
- Enter the highest degree you have earned or leave the field blank
- Enter two or three key words by which someone else might search for the study
- If you have an outside sponsorship, enter the name of the study’s sponsor. If not, leave this field blank.
- Leave the field for the “Internal Reference Number” blank.
- Select Continue
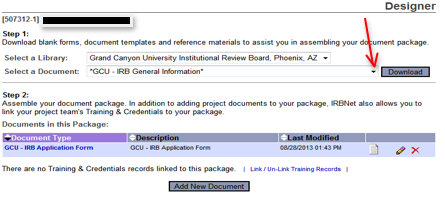
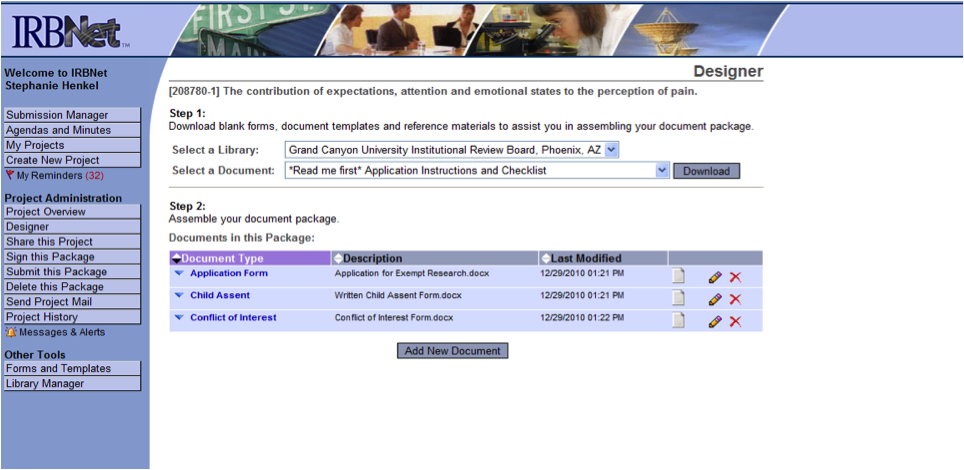
- You will be brought to a screen called Designer. This is where you can access the library of forms in the Forms and Templates area. You can download any document to your hard drive so you can edit and save the form(s) and then attach it to the study. At this point, you will see a message stating there are no documents in this study.
- Step 1: Click on Add New Document to begin the application and document loading process. (see below)
- Step 2: Select Click on Add to start the GCU – IRB Application Form
- Step 3: You will then proceed to follow each screen to guide you through the application. Please ensure you complete EVERY question, if it does not apply either write/mark “NA” or explain why it does not apply.
- Once you have completed the Smart Form GCU IRB Application, click on “Save and Exit”, you will then be brought to the Designer page again to load the remaining documents for IRB / AQR review.
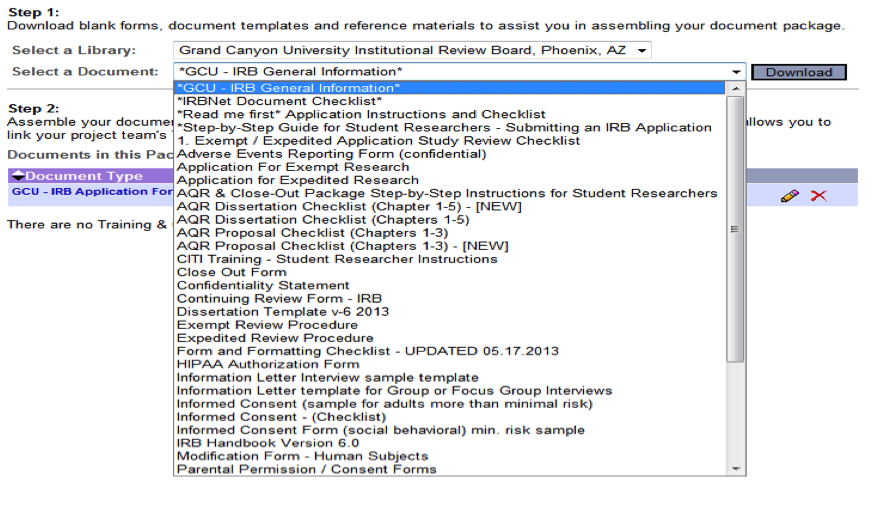
- Step 4: Click on the drop-down menu to the right of Select a Document to complete the necessary forms (i.e., IRB Application, Consent Forms, Templates, etc.)
- Once you have selected a document, click on Download (see options below)
- Save the document to your own computer
- Complete each required form(s), using Microsoft Word. (You do not need to be logged in to IRBNet to complete the form once you have saved it to your own computer.)
- When ready, you may return to IRBNet to log in and load all required documents. If you have changed information about the study, such as the title, click (Edit) to make revisions; then click Save after you have made the revisions.
- Click on your Study's Title
- Click on Study Designer
- Under “Step 2” click on Add New Document
- Select the Document Type from the drop-down menu (i.e., Application Form, Consent Form, Letter, or Other)
- Type a description in the field (ie: Informed Consent form, D-30, xxx School Site Authorization)
- Click on Browse to upload your completed document (MS Word documents only)
- Select Attach
- To attach other documents (i.e., dissertation proposal, permission letters) complete steps 22 through 26
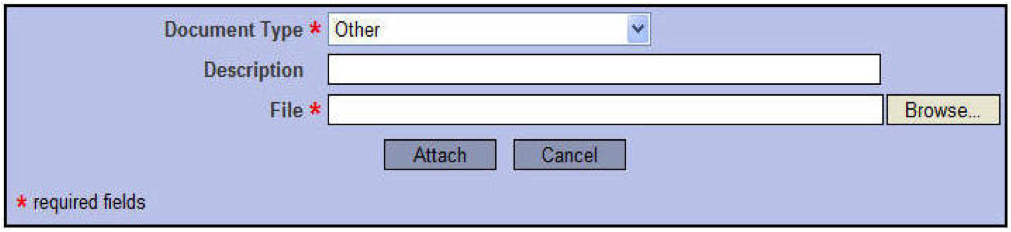
NOTE: Please save scanned images as black and white. Please do not scan images in color or grayscale. Whenever possible, request an electronic version (i.e. PDF) of an approval letter or statement.
Share a Project / Package
- Log in to www.IRBNet.org (if not already logged in)
- Select your Study’s Title
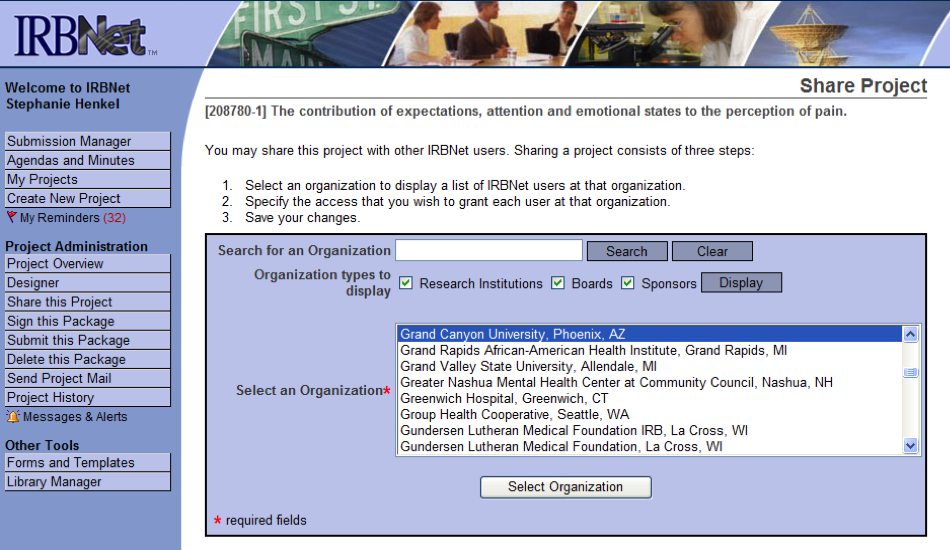
- From the menu at the left of the screen, click on Share this Project
- When the “Share Project” screen appears, click on the word Share
- Select Grand Canyon University, Phoenix, AZ from the list and drop-down menu
- Click on Select Organization
- On the next screen, type in your Chair’s last name in the search field (see below)
- Click on Search
- When your Chair’s name appears, choose Full as the type of “Permission Access” you are granting to the Chair. Listed below are the permissions available for each person. Keep in mind what access they will need.
- Type in any comments you wish to send to your Chair’s in the comments field
- Click on Save (please see below).


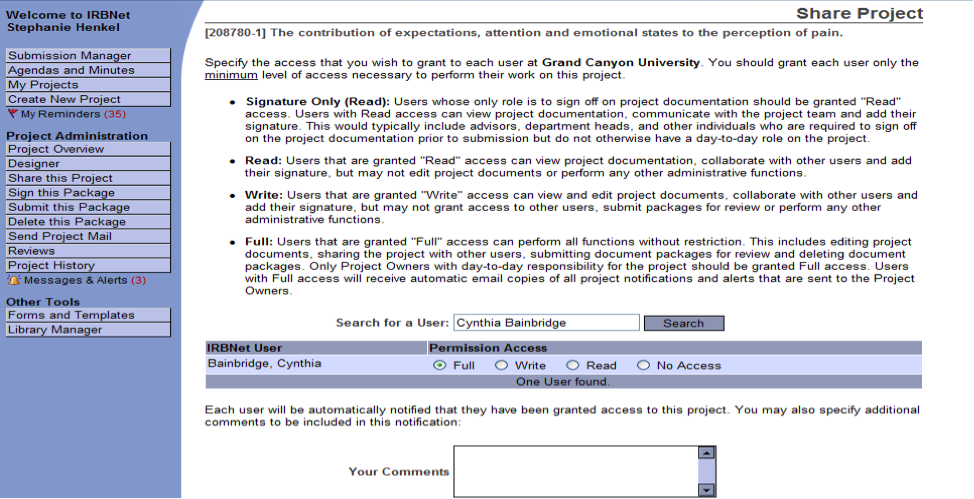
NOTE: The system will automatically notify your Chair that the project is ready for his or her review, signature, and submission. If the Chair deems the application complete and ready for review, s/he will submit the application for IRB review as appropriate.
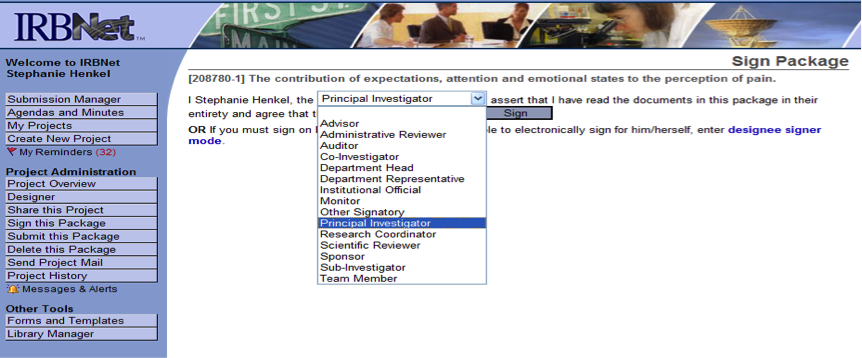
Sign this Package
- Log in to www.IRBNet.org (if not already logged in)
- Select your Study’s Title
- The next step is for the researcher to sign the package.
- From the menu/toolbar at the left of the screen, click on Sign this Package (see below)
- When the signature page appears, select Principal Investigator from the drop-down menu.
- Click Sign
Submit Package: ONLY CHAIRS CAN SUBMIT packages. This next step is for CHAIRS ONLY!!
This next step is for CHAIRS ONLY!! Please do not complete this step unless you are a chair; if done so the project will be rejected and sent back.
Chairs: Once you receive an email notification from the learner indicating the IRB project is ready, please review the project and documentation. If all required documentation is ready and is approved, then you will submit the project to the IRB.
- Log in to www.IRBNet.org (if not already logged in)
- Select your Study’s Title
- The next step is for the researcher to sign the package.
- From the menu/toolbar at the left of the screen, click on Sign this Package (see below)
- When the signature page appears, select Advisor from the drop-down menu.
- Click Sign
- Search and find Grand Canyon University IRB
- Chairs will select a Submission Type (SEE OPTIONS BELOW) …
- If this is the first submission for the learner, select New Project
- If submission needs a modification to the project, then you will select Amendment/Modification
- If submission needs to be revised, then you will select Revision
- If submission is the learners final submission to the IRB for the dissertation phase, then you will select Closure/Final Report
- If the submission / project is an Expedited or Full Review, will take longer than one year and if the year end is approaching, and if the learner is still collecting data or doing research, then you will select Continuing Review/Progress Report
- If submission was funded or was provided a grant, then you select Funding/Grant
- If submission had an unanticipated problem (UP), then you will select Unanticipated Problem (UP)
- If submission had an adverse event, then you will select Adverse Event (non-UP)
- If submission had a deviation or a violation, then you will select Protocol Deviation/Violation
- If submission needs another form of reportable event, then you will select Other Reportable Event
- If submission needs a publication, then you will select Publication
- If submission is a response or follow-up based on the projects information, then you will select Response/Follow-Up
- If submission type is something other than what is listed above, then select Other
- Chairs will then click Submit
Add or Revise Documents (Modifications / Continuation Required)
If you have submitted a package; and the IRB Chair/Board deems “Modifications are Required”, you will assemble your documents and chair will submit the revision.
- Log in to www.IRBNet.org (if not already logged in)
- Select the Title of the Study you wish to access
- Click on the Designer button
- Follow the steps below to add or update documents into the project:
- Select the Document Type from the drop-down menu under Step 2 that best describes the item you want to attach. (Use “Other” if none of the exact names fits the document type.)
- Type a description of the document
- Upload the file you wish to attach
- Select Attach
- Repeat the bulleted steps above for all the documents that apply to your project.
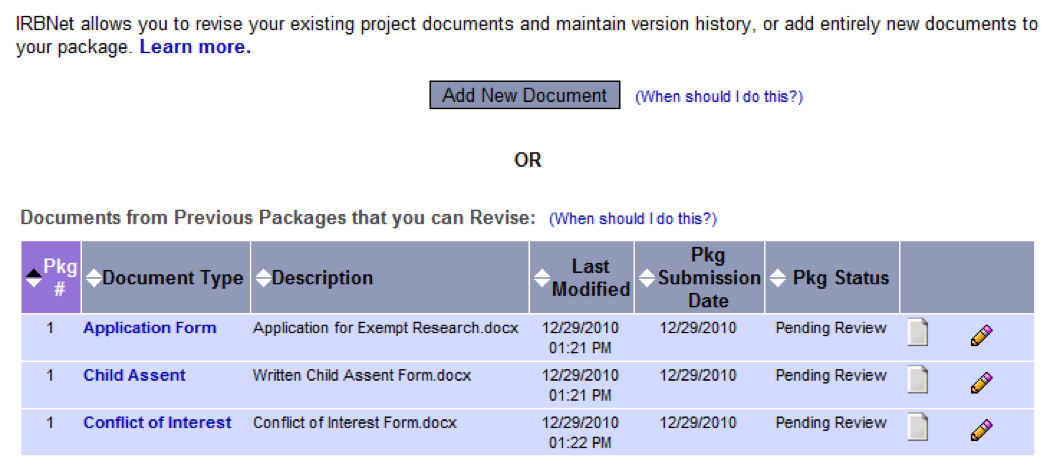
- From the list of Documents from Previous Packages at the bottom of the page, download the document to be revised by clicking on the Document Type or on the paper icon which will allow you to Update that file. (see below)
- When you have uploaded all required documents, please do not click on “Submit this Package.” Instead, send an email to your Chair indicating your revisions have been made. Then the Chair will submit the revision.
- If a study has been deferred and the revisions have been made, both the student researcher and Chair must sign the submission again.
NOTE: In general, student researchers should upload all documents required for the package. However, only your Chair can submit and resubmit the project.
Documents Required for IRB Review
- Online IRB Study Application
- Research Prospectus Approval Form (GCU D-20)
- AQR/Proposal Approval Form (GCU D-35)
- Approved Research Prospectus (ALL editing complete)
- Approved Post-Defense Proposal (ALL editing complete)
- Student Researcher CITI training certifications (2)
- Human Research
- RCR (Responsible Conduct Research)
- Recruiting Materials (advertisements, cover letters, scripts, etc.)
- Supporting documents*
- Recruitment Materials*
*This may also include approval letters, site authorization approval, authorization request letter (s), consent / assent forms (templates), surveys, questionnaires, etc.
**NOTE: Please download the IRBNet Document Checklist from the forms and templates tab in IRBNet to ensure you have all of the required documents for IRB review.
Documents Required for Continuing Review
All continuing review submissions must include the following documents for review:
- Continuing Review form
- Original IRB Application
- IRB approval notice / letter
- Current draft of the dissertation proposal (edits complete)
- Copy of the Informed Consent / Assent document(s)—if applicable
Documents Required for Modification to Project after IRB approval
All modification/revision submissions must include the following documents for review:
- Modification form
- Revised IRB Application (reflecting the requested revisions)
- IRB approval notice / letter
- Current draft of the dissertation proposal (edits complete)
- Copy of all revised documents (i.e., informed consent form (s), advertisements, proposal, letters, etc.)
- Copy of permission letters from the research site(s)—if applicable
Retrieve Board Documents
- Log in to www.IRBNet.org
- Click on the title of the study you wish to access
- Scroll down to “Submitted to:”
- Click on Review Details
- Under “Board Documents” click on the document name(s) or the(paper) icon to view the document(s)
Check Status of IRB Application
NOTE: The status indicator will give you information on the status of an IRB Application. Please read through the following indicators. Any time the status is changed, the student researcher and the Chair will receive an email notification from IRBNet.
- Work in Progress: The study has been created but has not been submitted to the Grand Canyon University IRB for review. Once the application is ready for review, the Chair is the only individual who should “submit” a student researchers’ package to the Grand Canyon University IRB. Grand Canyon University
- Pending Review: The study/package has been submitted to Grand Canyon University IRB and the Submission Coordinator is screening the study/package for completeness.
- Modifications Required or Information Required: If the submission is incomplete (after being submitted) there will be an Incomplete Application Memo in the study stating what must be uploaded or modified in the study/package. Follow the steps on page 10 (How to Add or Revise Documents).
- Forwarded: If the submission is complete, the study is “forwarded” to the IRB reviewer for review. You and your Chair will receive an email from IRBNet once the evaluation is complete.
- Approved: The study is approved by the Grand Canyon University IRB. A letter of Approval and the completed evaluation form will be located in the study.
- Deferred: The study has been deferred for either minor or major revisions. Please review the evaluation form for further information. It is located in the study on IRBNet.
- Withdrawn: The study has been withdrawn from review for different reasons, e.g., multiple submissions.
If you are checking the status of Level 2 & 3 review first check with the chair, second, check learner’s unique LDP page located in DC Network.